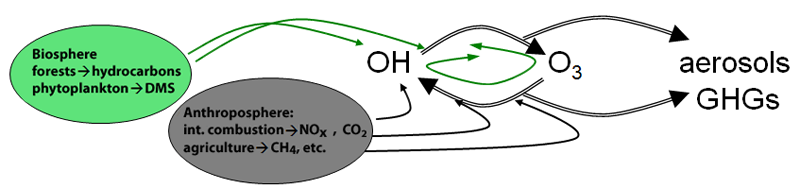
Atmospheric photochemical oxidation is a crucial component of the Earth’s climate. The above cartoon crudely illustrates how the processes are linked. Gases of broadly variable reactivity (from CH4 with a lifetime of ~10 yrs to isoprene with a lifetime of ~10 minutes) are emitted to the atmosphere and systematically removed by oxidation, predominantly overseen by the photochemical agent – OH, the hydroxyl radical. Photochemical oxidation renders products that are generally more polarizable and water soluble and thus more easily removed from the atmosphere by precipitation and turbulent transport to the surface (wet and dry deposition, respectively.) Some of the less volatile components can spawn and grow aerosols, which along with IR-active gases, can couple to the radiation field and influence the energy balance of the Earth directly.
Some of our air quality work includes the investigation of hydrocarbon oxidation above Blodgett forest on the western slope of the Sierra Nevada, using measurements of gas phase formaldehyde (HCHO) during the first and second BEARPEX (Biosphere Effects on Aerosols and Photochemistry) Experiments of 2007 and 2009. We have also investigated CO emissions from coastal waters and shipping offshore of the Bodega Marine Lab.
In conjunction with the Air Quality Research Center at UC Davis, we are currently establishing a climate/air quality monitoring stations on Cahto Peak in the Angelo Coast Range Reserve, and at Bodega Bay to investigate the inflow of exogenous air pollutants to North America.